Alkaline battery
Alkaline battery
Most alkaline batteries are primairy batteries and can be used only once. There is a development to make alkaline batteries rechageable.As in a Zinc carbon battery the ingredients are similar; zinc, manganese-oxide in carbon.
But not in an acid environment like ammonium chloride (pH=5) but in a caustic environment with potassium hydroxide (pH>8 )
Compared with the Zinc carbon battery the alkaline battery has a longer shelf life and a higher energy density.
Energy density is the amount of energy per mass of the battery. Because there are different sizes of a battery like AAA size, the energy density per chemistry type, in this case alkaline battery is the same.
But if you compare with other electro-chemistry type like Zinc-carbon vs Alkaline the energy density is different
Alkaline batteries are not as prone to leak as the Zinc carbon battery but when not used for a long tie they will leak.
The disadvantage of an alkaline battery is its high internal resistance.
Advantages of alkaline batteries
Long battery life with a fantastic shelf life.There are few types of batteries on the market today that have both the shelf life and the battery life of alkaline batteries -- especially when it comes to the devices they are powering. A long shelf life means that an alkaline battery will hold its charge even if you don't use it for years after its manufacturing. This is a big difference from other types of batteries which are recommended to be opened and used or charged at least once within the first year of purchase. The average alkaline battery can last upwards of seven years, sometimes even more without ever being used, and will only lose five percent of its energy by doing so. And then when you do use them, the alkaline battery will power your devices for months on end even with regular use. Don't believe us on this? Just consider when the last time was that you changed your remote's batteries. Safe to handle and use.
Many batteries are dangerous and must be handled with extreme care. Take lead and acid-based batteries, which can have severe health repercussions if they are destroyed and those materials are touched. In contrast, alkaline batteries, while it obviously isn't recommended to open them up, their hazardous materials are much harder to reach with a much lower susceptibility to leakage. This makes them much safer to handle and use. High energy density.
One of the primary competitors of the alkaline battery is the zinc-carbon battery, but alkaline batteries far and away outperform them thanks to their high energy density. In fact, the average alkaline battery will have double the energy density of a similarly sized zinc-carbon battery. This high density enables the alkaline battery to produce the same amount of energy for twice the period of time. Ability to operate across temperatures. Another frustrating thing about other types of batteries is that they often don't work well or will even outright fail in extreme temperatures. Alkaline batteries are not like this. The alkaline battery will operate well both in hot and cold temperatures without users noticing any type of impact in its performance. This is why alkaline batteries are the most recommended type of battery to use in outdoor equipment like flashlights, walkie talkies, mosquito repellant devices, and more.
Leaks Over time, alkaline batteries are prone to leaking potassium hydroxide, a caustic agent that can cause respiratory, eye and skin irritation. This can be avoided by not mixing different battery types in the same device, replacing all of the batteries at the same time, storing in a dry place, and removing batteries from devices for storage.
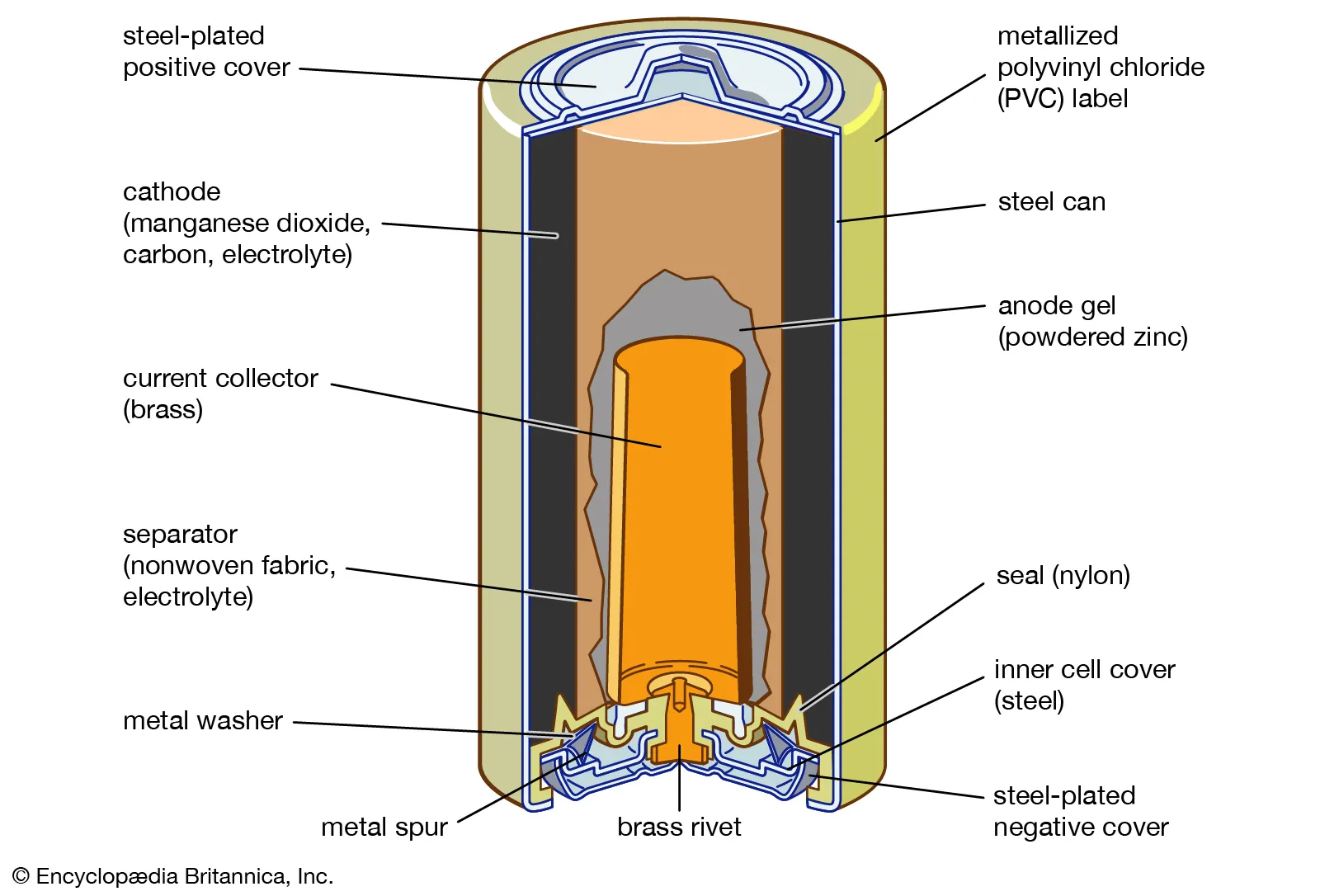
Battery anatomy
Development of the alkaline battery (history)
Development of Alkaline Batteries Alkaline batteries were invented in the early twentieth century. Important people associated with the creation, development, and spread of the alkaline battery include: Ernst Waldemar Jungner: In March 1899, he created the first patent for alkaline batteries. He envisioned that they would be used mainly in circumstances that required a large amount of consistent energy. This patent was given in Sweden. Georg Neumann: Neumann was a French scientist who created the first alkaline sealed cell in 1947 in Paris during his work for the Bureau Technique Gautrat. Alred Landau: He created the first iterations of the Rayovac batteries in 1906. Rayovac lithium batteries were first marketed in 1949. Lewis Urry: In 1949, this inventor created the Eveready alkaline battery. Although this battery was not immediately accepted by consumers, after several years he became successful. Thirty years after its invention, the battery and the company that made it changed their names to Energizer.
Capacity Unlike NiMH rechargeable batteries, alkaline batteries are normally not sold with a nominal capacity. Alkalines have a high internal resistance, and a high thermal coefficient of resistivity - the faster an alkaline battery is drained, the higher percentage of the load it dissipates as heat. Therefore, the capacity of an alkaline battery is strongly dependent on the load, even at moderate loads. A AA-sized alkaline battery might have an effective capacity of 3000 mAh at low power, but at a load of 1000 mA, which is common for digital cameras, the capacity could be as little as 700 mAh.[2] Current The amount of current an alkaline battery can deliver is roughly proportional to its physical size. This is a result of decreasing internal resistance as the internal surface area of the cell increases. A general rule of thumb is that an AA alkaline battery can deliver 1000mA without any significant heating. Larger cells, such as C and D cells, can deliver more current. Applications requiring high currents of several amps, such as high powered flashlights and boom-boxes, will require D sized cells to handle the increased load. Volume for volume, alkaline batteries have inferior current handling capacity when compared to other chemistries like NiCd and NiMH. However, alkaline batteries cost significantly less.
5.4 Effect of Temperature The alkaline-manganese dioxide system is best suited for use over a temperature range of -4°F to 130°F (-20°C to 54°C). At lighter loads, some output can be obtained at temperatures as low as -20°F( 30°C). Actual service depends on cell size and current drain. For most cells, up to 75 percent of the rated capacity at room temperature can be delivered at 32°F(0°C). The graph in Figure 8 illustrates how cell size and current drain affect performance over a range of temperatures. As current drain increases, temperature impact becomes more dramatic. Low temperature 5.5 Internal Resistance Alkaline cells, because of their compact construc tion and highly conductive electrolyte, have low internal resistance, usually less than 1 ohm. The low internal resistance characteristic is a benefit in applications - involving high current pulses. Unlike regular zinc-carbon cells, alkaline cells do not require rest periods between pulses and maintain their low internal resistance, increasing only at the very end of useful life.
Electrochemistry
The half-reactions are:
Zn(s) + 2OH−(aq) ⇌ ZnO(s) + H2O(l) + 2e− [E oxidation° = +1.28 V]
2MnO2(s) + H2O(l) + 2e− ⇌ Mn2O3(s) + 2OH−(aq) [E reduction° = +0.15 V]
Overall reaction:
Zn(s) + 2MnO2(s) ⇌ ZnO(s) + Mn2O3(s) [e° = +1.43 V]
Primary batteries like the alkaline battery are very popular. On a yearly base about 10 billion are sold.

